Multiple AAV Serotypes Market 2026 Set to Soar as Gene Therapy Innovation and Global Biotech Investments Accelerate
This report, published by Towards Healthcare, a sister firm of Precedence Research, provides an in-depth analysis of the global multiple AAV serotypes market, highlighting key trends, growth drivers, and future opportunities. It offers comprehensive insights into the evolving landscape of gene therapy advancements and biotechnological innovations shaping the market.
Ottawa, Nov. 11, 2025 (GLOBE NEWSWIRE) — The multiple AAV serotypes market is witnessing robust expansion, with revenues projected to reach several hundred million dollars by 2034, growing steadily throughout the 2025–2034 forecast period. This upward trajectory is fueled by increasing applications across biotechnology and healthcare sectors.
The rising prevalence of cancer, hemophilia, muscular dystrophy, and other rare genetic disorders is driving greater demand for AAV-based therapies. Additionally, substantial investments in biotechnology and advancements in AAV production technologies across China, Japan, and South Korea are contributing to market growth. The industry is also shifting toward engineered and hybrid AAV serotypes, a trend actively supported by leading CDMOs. The widespread adoption of diverse AAV serotypes in developing next-generation and more effective gene therapies continues to be a key growth catalyst for the global multiple AAV serotypes market.
The Complete Study is Now Available for Immediate Access | Download the Sample Pages of this Report @ https://www.towardshealthcare.com/download-sample/6248
Key Takeaways
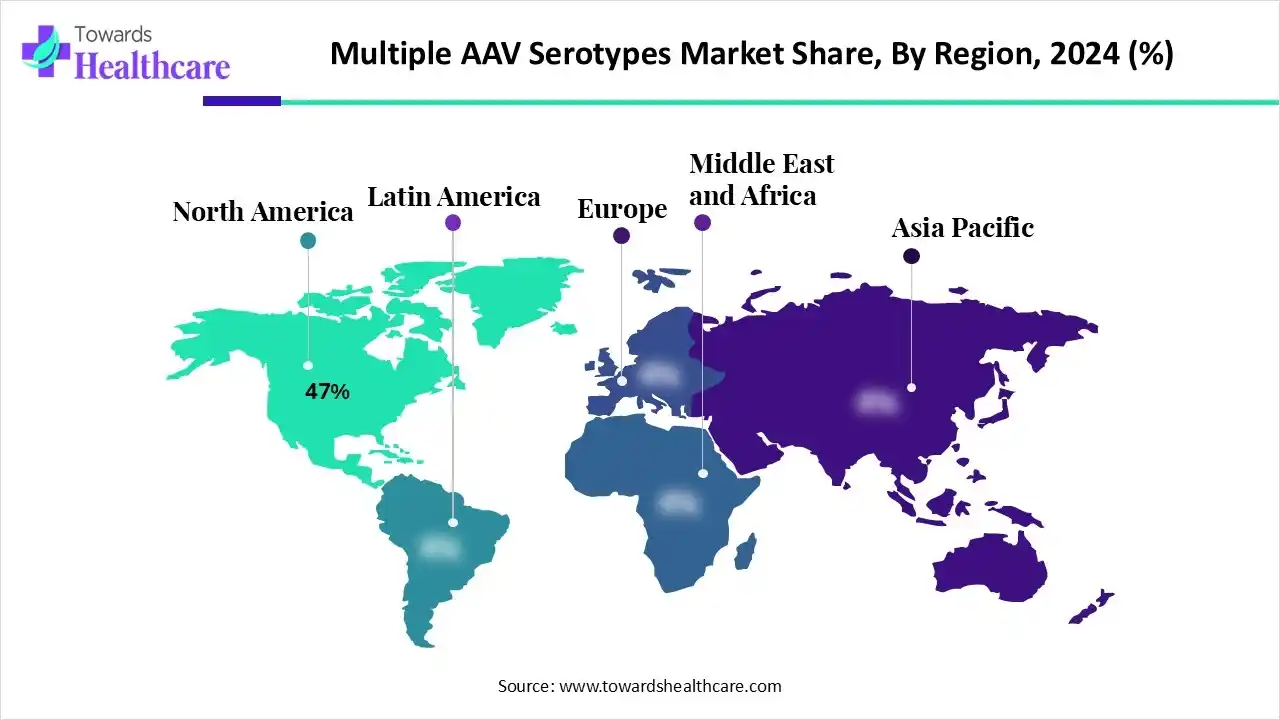
- North America captured a nearly 47% revenue share of the market in 2024.
- Asia Pacific is expected to grow at the fastest CAGR during the forecast period.
- By serotype, the AAV2 segment led with an approximate 31% share of the multiple AAV serotypes market in 2024.
- By serotype, the AAV9 segment is expected to witness rapid expansion in the studied years.
- By application, the neurology segment held a major share of approximately 39% of the market in 2024.
- By application, the infectious diseases segment is expected to grow at the fastest CAGR during 2025-2034.
- By technology/vector design, the self-complementary AAV (scAAV) segment dominated with nearly 44% revenue share of the market in 2024.
- By technology/vector design, the engineered/hybrid serotypes segment is expected to grow at the fastest CAGR in the coming years.
- By end user, the biotechnology & pharmaceutical companies segment held the biggest share of nearly 48% of the multiple AAV serotypes market in 2024.
- By end user, the contract development & manufacturing organisations (CDMOs) segment is expected to witness the fastest CAGR during 2025-2034.
Market Overview & Potential
The multiple AAV (Adeno-Associated Virus) serotypes market involves the development, production, and use of various AAV serotypes in gene therapy and research. The presence of multiple AAV serotypes improves therapeutic accuracy, fosters innovation in capsid engineering, and broadens the range of clinical uses in genetic diseases, rare conditions, and regenerative medicine. AAV vectors are among the most common delivery tools because of their low pathogenicity, ability to transduce both dividing and non-dividing cells, and potential to enable long-term gene expression.
You can place an order or ask any questions, please feel free to contact us at [email protected]
What Is The Growth Potential Responsible For The Growth Of The Multiple AAV Serotypes Market?
The market for multiple AAV serotypes is driven by the increasing success of gene therapies, which require different serotypes for specific tissue targeting. Key drivers include the need for greater specificity to overcome limitations like immune responses and off-target delivery, the growing number of clinical trials for various diseases, and the expansion of manufacturing to meet demand. The development of novel and engineered serotypes further fuels market growth by enabling more effective treatments and reaching new therapeutic areas.
What Are The Growing Trends Associated With The Multiple AAV Serotypes Market?
Discovery and engineering of novel serotypes:
- Computational design is being used to create engineered capsids with new tissue tropisms and to mitigate pre-existing immunity.
Manufacturing and production optimisation:
- A significant focus is on developing scalable, reproducible, and cost-effective manufacturing processes to meet growing demand.
Advancements in vector technology:
- Efforts are underway to increase the cargo capacity of AAV vectors to allow for the delivery of larger genes.
Gene therapy expansion:
- The increasing use of adeno-associated virus (AAV) vectors as the leading platform for gene therapy is the primary driver.
What Is The Growing Challenge In The Multiple AAV Serotypes Market?
Market challenges for AAV serotypes include difficulties in manufacturing and scaling up production, with issues like low viral particle yields and high costs. Other challenges are related to vector performance, such as limited tissue specificity, the inability to cross the blood-brain barrier, and immune responses from pre-existing antibodies. Additionally, there are analytical challenges, including a lack of standardisation, high cost of testing methods, and the difficulty in accurately quantifying full vs. empty capsids.
Become a valued research partner with us – https://www.towardshealthcare.com/schedule-meeting
Regional Analysis
How Did North America Dominate The Multiple AAV Serotypes Market In 2024?
North America captured a nearly 47% revenue share of the market in 2024. North America leads the multiple AAV serotypes market, driven by its advanced healthcare infrastructure, high gene therapy awareness, and strong government support for research and development. This dominance is supported by numerous clinical trials and key market players, with a high demand for AAV vectors for treating chronic diseases. AAV9 is currently a dominant serotype due to its efficacy, while the engineered/hybrid capsids segment is projected to see rapid growth, as they can overcome natural serotype limitations
What Made The Asia Pacific Significantly Grow In The Multiple AAV Serotypes Market In 2024?
Asia Pacific is expected to grow at the fastest CAGR during the forecast period. The market for multiple AAV serotypes in the Asia-Pacific region is experiencing significant growth, driven by increasing government support, rising prevalence of genetic disorders, and expansion of clinical research capabilities. China, Japan, and South Korea are leading this expansion through increased investment, government initiatives, and the development of cost-effective manufacturing, which is making the region a strategic hotspot for AAV gene therapies. Advancements in AAV vector technology fuel the market’s growth, the exploration of new serotypes, and the expansion of AAV vector applications.
Segmental Insights
By Serotype,
The AAV2 segment led with an approximate 31% share of the multiple AAV serotypes market in 2024. AAV2 is among the most established adeno-associated virus serotypes used in gene therapy due to its well-characterised genome and strong tropism for liver and retinal tissues. It has a long record of safety and efficacy in early gene therapy trials. Biotechnology firms are increasingly using AAV2 in ophthalmology and metabolic disorder applications owing to its predictable performance and scalable production systems.
The AAV9 segment is expected to witness rapid expansion in the studied years. AAV9 is highly preferred for its ability to cross the blood–brain barrier, making it ideal for neurological and neuromuscular gene therapy. It offers systemic transduction efficiency for the central nervous system (CNS) and cardiac targets. Demand is rapidly expanding in neurodegenerative disease programs such as SMA and ALS, driving investments from pharmaceutical companies in AAV9-based clinical and commercial production.
Get the latest insights on life science industry segmentation with our Annual Membership: https://www.towardshealthcare.com/get-an-annual-membership
By Application,
The neurology segment held a major share of approximately 39% of the market in 2024. Neurology represents the largest application area for multiple AAV serotypes due to the viruses’ ability to deliver genes to the brain and spinal cord. AAV9 and hybrid variants are particularly valuable for treating genetic neurological disorders like SMA and Parkinson’s disease. Increasing clinical success rates are accelerating pipeline expansion in Asia-Pacific and North America.
The infectious diseases segment is expected to grow at the fastest CAGR during 2025-2034. In infectious disease applications, AAV serotypes are employed as vaccine vectors and gene-based immunotherapies. AAV2 and engineered capsids are showing promise in expressing viral antigens with long-term immunogenicity. Global health initiatives are supporting R&D in AAV-based vaccines for HIV and hepatitis, enhancing collaborations between biotech firms and vaccine developers.
By Technology/Vector Design,
The self-complementary AAV (scAAV) segment dominated with nearly 44% revenue share of the market in 2024. Self-complementary AAV (scAAV) technology provides faster and more efficient transgene expression by bypassing the need for second-strand DNA synthesis. It is used in preclinical and early-stage therapies requiring rapid onset, such as neurological and ocular gene treatments. The technology improves efficacy but limits packaging capacity, driving innovation in capsid engineering.
The engineered/hybrid serotypes segment is expected to grow at the fastest CAGR in the coming years. Engineered and hybrid AAV serotypes are designed to improve tissue targeting, immune evasion, and yield. Advanced capsid libraries and directed evolution approaches have led to new variants with enhanced CNS or liver tropism. These custom serotypes are increasingly adopted by CDMOs and biopharma innovators to achieve patient-specific therapeutic performance.
By End User,
The biotechnology & pharmaceutical companies segment held the biggest share of nearly 48% of the multiple AAV serotypes market in 2024. Biotechnology and pharmaceutical companies dominate the use of multiple AAV serotypes for in-house therapeutic development. These firms focus on expanding AAV pipelines targeting rare genetic and neurodegenerative diseases. They increasingly employ AAV9, AAV2, and engineered variants, investing heavily in scalable vector manufacturing and regulatory compliance for clinical and commercial programs.
The contract development & manufacturing organisations (CDMOs) segment is expected to witness the fastest CAGR during 2025-2034. CDMOs are critical partners for AAV production and optimisation, offering expertise in large-scale vector manufacturing, analytical testing, and regulatory support. The demand for CDMO services has surged as biotech firms outsource AAV design, process development, and GMP-grade production. Hybrid serotype manufacturing is a major growth area for CDMOs in Asia-Pacific and North America.
Browse More Insights of Towards Healthcare:
The AAV manufacturing service market is rapidly advancing on a scale, with expectations of accumulating hundreds of millions in revenue between 2025 and 2034. Market forecasts suggest robust development fueled by increased investments, innovation, and rising demand across various industries.
The global AAV vector CDMO services market size is calculated at USD 1.24 billion in 2024, grew to USD 1.43 billion in 2025, and is projected to reach around USD 5.14 billion by 2034. The market is expanding at a CAGR of 15.24% between 2025 and 2034.
The AAV gene therapy market size was estimated at US$ 2.75 billion in 2024, projected to increase to US$ 3.85 billion in 2025 and reach US$ 78.56 billion by 2034, showing a healthy CAGR of 40.1% across the forecast years.
Recent Developments
- In September 2025, Abselion, a pioneering life sciences technology company, introduced the AAVX Total Capsid Quantification Kit and the AAV9 Total Capsid Quantification Kit.
- In August 2025, ProBio, a contract development and manufacturing organisation (CDMO), launched current good manufacturing practice (CGMP) adeno-associated virus (AAV) manufacturing services in Hopewell, N.J.
- In October 2024, Isolere Bio by Donaldson, a groundbreaking bioprocessing technology provider, unveiled research-grade IsoTag™ AAV reagent for the purification of adeno-associated viral vectors (AAV).
Multiple AAV Serotypes Market Key Players List
- Audentes Therapeutics (Astellas)
- Biomarin Pharmaceutical
- Pfizer Inc.
- Novartis AG
- CSL Behring
- WuXi Advanced Therapies
- Thermo Fisher Scientific
- Catalent Gene Therapy
- Lonza Group
- Oxford Biomedica
- MeiraGTx Holdings
- Abeona Therapeutics
- GenSight Biologics
- 4D Molecular Therapeutics
- LogicBio Therapeutics (Alexion/AstraZeneca)
Segments Covered in The Report
By Serotype
- AAV2
- AAV8
- AAV9
- AAV1
- AAV5
- AAVrh10
- Other Engineered/Next-gen Capsids
By Application
- Neurology
- Ophthalmology
- Musculoskeletal/Metabolic Disorders
- Hematology
- Cardiology
- Infectious Diseases
- Others (Dermatology, Rare Genetic Disorders)
By Technology/Vector Design
- Naturally Occurring Serotypes
- Engineered/Hybrid Serotypes
- Self-complementary AAV (scAAV)
- Others (Synthetic Promoter Integration, MicroRNA Regulation)
By End User
- Biotechnology & Pharmaceutical Companies
- Academic & Research Institutions
- Contract Development & Manufacturing Organisations (CDMOs)
- Hospitals & Specialized Gene Therapy Centers
By Region
- North America
- U.S.
- Canada
- Asia Pacific
- China
- Japan
- India
- South Korea
- Thailand
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Sweden
- Denmark
- Norway
- Latin America
- Brazil
- Mexico
- Argentina
- Middle East and Africa (MEA)
- South Africa
- UAE
- Saudi Arabia
Immediate Delivery Available | Buy This Premium Research @ https://www.towardshealthcare.com/checkout/6248
Access our exclusive, data-rich dashboard dedicated to the healthcare market – built specifically for decision-makers, strategists, and industry leaders. The dashboard features comprehensive statistical data, segment-wise market breakdowns, regional performance shares, detailed company profiles, annual updates, and much more. From market sizing to competitive intelligence, this powerful tool is one-stop solution to your gateway.
Access the Dashboard: https://www.towardshealthcare.com/access-dashboard
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics, with a strong emphasis on life science research. Dedicated to advancing innovation in the life sciences sector, we build strategic partnerships that generate actionable insights and transformative breakthroughs. As a global strategy consulting firm, we empower life science leaders to gain a competitive edge, drive research excellence, and accelerate sustainable growth.
You can place an order or ask any questions, please feel free to contact us at [email protected]
Europe Region: +44 778 256 0738
North America Region: +1 8044 4193 44
APAC Region: +91 9356 9282 04
Web: https://www.towardshealthcare.com
Our Trusted Data Partners
Precedence Research | Statifacts | Towards Packaging | Towards Automotive | Towards Food and Beverages | Towards Chemical and Materials | Towards Consumer Goods | Towards Dental | Towards EV Solutions | Nova One Advisor | Healthcare Webwire | Packaging Webwire | Automotive Webwire | Nutraceuticals Func Foods | Onco Quant | Sustainability Quant | Specialty Chemicals Analytics
Find us on social platforms: LinkedIn | Twitter | Instagram | Medium | Pinterest
 Disclaimer: The above press release comes to you under an arrangement with GlobeNewswire. DailyIndiaNews.com takes no editorial responsibility for the same.
Disclaimer: The above press release comes to you under an arrangement with GlobeNewswire. DailyIndiaNews.com takes no editorial responsibility for the same.


