Diabetic Neuropathy Clinical Trial Pipeline Gains Momentum: 18+ Companies Lead the Charge in Pioneering New Treatments | DelveInsight
Diabetic neuropathy is a common, progressive complication of diabetes characterized by nerve damage that leads to chronic pain, sensory loss, and functional impairment. The rising global prevalence of diabetes, which is expanding the patient pool and long-term disease burden is amplifying unmet need and accelerating demand for novel, disease-modifying, and non-opioid therapies due to the limited efficacy and tolerability of existing treatments.
New York, USA, Jan. 20, 2026 (GLOBE NEWSWIRE) — Diabetic Neuropathy Clinical Trial Pipeline Gains Momentum: 18+ Companies Lead the Charge in Pioneering New Treatments | DelveInsight
Diabetic neuropathy is a common, progressive complication of diabetes characterized by nerve damage that leads to chronic pain, sensory loss, and functional impairment. The rising global prevalence of diabetes, which is expanding the patient pool and long-term disease burden is amplifying unmet need and accelerating demand for novel, disease-modifying, and non-opioid therapies due to the limited efficacy and tolerability of existing treatments.
DelveInsight’s ‘Diabetic Neuropathy Pipeline Insight 2025‘ report provides comprehensive global coverage of pipeline therapies for diabetic neuropathy across various stages of clinical development. The report offers an in-depth analysis of key trends, emerging therapies, and competitive landscape dynamics, highlighting the strategies of major pharmaceutical companies to advance the pipeline and capitalize on future growth opportunities. In addition, it includes critical insights into clinical trial benchmarking, partnering and licensing activities, and regulatory pathways involving the FDA and EMA, enabling stakeholders to make informed decisions and optimize development strategies within the diabetic neuropathy domain.
Diabetic Neuropathy Clinical Trial Analysis Summary
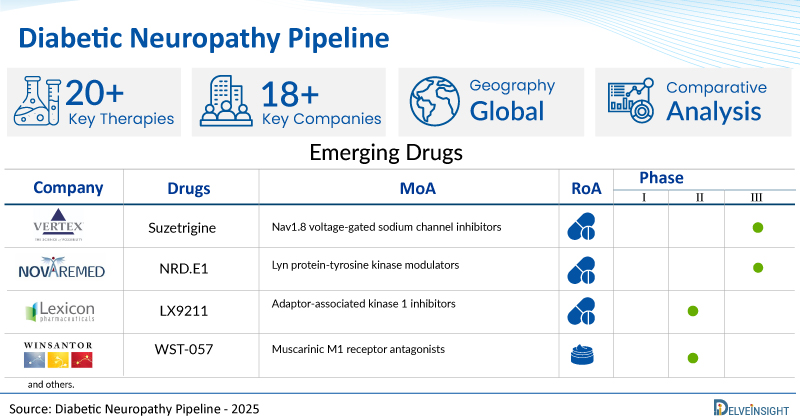
- DelveInsight’s diabetic neuropathy pipeline report depicts a robust space with 18+ active players working to develop 20+ pipeline diabetic neuropathy drugs.
- Key diabetic neuropathy companies such as Vertex Pharmaceuticals Incorporated, Novaremed, Trevena, Inc., WinSanTor, Inc., AlgoTherapeutix, Tris Pharma, Inc., JMackem Co., Ltd, Lexicon Pharmaceuticals, Merz Pharmaceuticals GmbH, and others are evaluating new diabetic neuropathy drugs to improve the treatment landscape.
- Promising pipeline diabetic neuropathy therapies, such as Suzetrigine, NRD.E1, TRV045, WST-057, ATX01, Cebranopadol, AJH-2947, LX9211, NT 201, and others, are in different phases of diabetic neuropathy clinical trials.
- Approximately 10+ diabetic neuropathy drugs are in the mid stage of development, whereas 2+ drugs are in the early stages of development.
- Notable MoAs in diabetic neuropathy clinical trials include Nav1.8 voltage-gated sodium channel inhibitors, Lyn protein-tyrosine kinase modulators, Sphingosine 1 phosphate receptor modulators, Adaptor-associated kinase 1 inhibitors, Muscarinic M1 receptor antagonists, Voltage-gated sodium channel modulators, and others.
Request a sample and discover the recent advances in diabetic neuropathy drugs @ Diabetic Neuropathy Pipeline Report
What is Diabetic Neuropathy?
Diabetic peripheral neuropathy (DPN) is the most prevalent type of peripheral nerve disorder and a significant complication of diabetes, resulting from sustained elevations in blood glucose levels that cause progressive nerve injury. It commonly manifests as numbness, tingling, burning, pain, and muscle weakness, predominantly in the lower extremities. As the condition advances, patients may lose protective sensation, substantially increasing the risk of foot ulcers, infections, and eventual amputations. Diagnosis is primarily based on clinical evaluation, including patient history and sensory examination, while specialized diagnostic tests are generally reserved for atypical presentations. Treatment strategies emphasize optimal glycemic control, patient education, routine foot care, and symptomatic management of neuropathic pain to minimize complications.
Diabetic neuropathy encompasses a broad spectrum of clinical manifestations depending on the nerves involved. Peripheral neuropathy, the most frequent subtype, typically begins with sensory disturbances such as pain, numbness, tingling, or burning in the toes and feet, gradually spreading proximally and, in some cases, affecting the hands and arms. Patients may also develop muscle weakness, diminished reflexes, heightened sensitivity to touch, and an increased susceptibility to foot injuries, ulcers, and infections due to sensory loss. Autonomic neuropathy disrupts involuntary bodily functions, leading to gastrointestinal symptoms (including bloating and constipation), cardiovascular abnormalities such as orthostatic hypotension, urinary and sexual dysfunction, and irregular sweating. Less common forms include proximal neuropathy, characterized by pain and weakness in the hips or thighs, and focal neuropathy, which presents as sudden, localized pain or nerve impairment in specific regions such as the torso, face, or head.
The underlying mechanisms of DPN are multifactorial and involve both metabolic and vascular processes initiated by chronic hyperglycemia. Persistently elevated glucose levels promote insulin resistance, lipid abnormalities, and mitochondrial and endoplasmic reticulum oxidative stress, resulting in excessive production of reactive oxygen species (ROS) and inflammatory responses. This pro-oxidative state facilitates macrophage infiltration into peripheral nerves, where the release of inflammatory mediators contributes to nerve fiber damage. Additional pathogenic factors include the accumulation of advanced glycation end products (AGEs) and dysregulation of metabolic pathways such as the polyol, protein kinase C, and hexosamine pathways. Compromised neurovascular integrity, endothelial dysfunction, and impaired nerve regeneration further accelerate progressive nerve degeneration.
Find out more about diabetic neuropathy drugs @ Diabetic Neuropathy Treatment
A snapshot of the Pipeline Diabetic Neuropathy Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| Suzetrigine | Vertex Pharmaceuticals Incorporated | III | Nav1.8 voltage-gated sodium channel inhibitors | Oral |
| NRD.E1 | Novaremed | II | Lyn protein-tyrosine kinase modulators | Oral |
| LX9211 | Lexicon Pharmaceuticals | II | Adaptor-associated kinase 1 inhibitors | Oral |
| WST-057 | WinSanTor | II | Muscarinic M1 receptor antagonists | Topical |
| ATX01 | AlgoTherapeutix | I | Voltage-gated sodium channel modulators | Topical |
| TRV045 | Trevena, Inc. | Preclinical | Sphingosine 1 phosphate receptor modulators | Oral |
Learn more about the emerging diabetic neuropathy therapies @ Diabetic Neuropathy Clinical Trials
Recent Developments in Diabetic Neuropathy Treatment Space
- In October 2025, Lexicon Pharmaceuticals, Inc. announced the Company presented additional clinical data and program updates from its Phase II pilavapadin program at the 19th Annual Pain Therapeutics Summit. These data follow the topline results from the Phase IIb PROGRESS study of pilavapadin in diabetic peripheral neuropathic pain (DPNP).
- In September 2025, Novaremed AG announced the completion of the last patient last visit (LPLV) in the National Institutes of Health (NIH)-funded Phase IIb EN21-01 trial. The trial evaluates Novaremed’s non-opioid investigational drug nispomeben for the oral treatment of chronic pain associated with painful diabetic peripheral neuropathy (DPN).
- In May 2025, Novaremed AG announced that the recruitment is completed in the National Institutes of Health (NIH) -sponsored Phase IIb EN21-01 trial. The study evaluates NRD.E1 for the treatment of chronic pain associated with diabetic peripheral neuropathy.
- In October 2024, Sonnet BioTherapeutics Holdings, Inc. announced that it has entered into a licensing agreement with Alkem Laboratories Limited for the research, development, manufacturing, marketing and commercialization of its molecule SON-080 for the treatment of diabetic peripheral neuropathy (DPN) in India.
- In July 2024, NeuroBo Pharmaceuticals, announced the signing of an exclusive license agreement, providing MThera Pharma Co., Ltd. (MTHERA) with the rights to NB-01 for the treatment of painful diabetic neuropathy.
Scope of the Diabetic Neuropathy Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Oral, Intravenous, Subcutaneous, Parenteral, Topical
- Therapeutics Assessment By Molecule Type: Recombinant fusion proteins, Small molecule, Monoclonal antibody, Peptide, Polymer, Gene therapy
- Therapeutics Assessment By Mechanism of Action: Nav1.8 voltage-gated sodium channel inhibitors, Lyn protein-tyrosine kinase modulators, Sphingosine 1 phosphate receptor modulators, Adaptor-associated kinase 1 inhibitors, Muscarinic M1 receptor antagonists, Voltage-gated sodium channel modulators.
- Key Diabetic Neuropathy Companies: Vertex Pharmaceuticals Incorporated, Novaremed, Trevena, Inc., WinSanTor, Inc., AlgoTherapeutix, Tris Pharma, Inc., JMackem Co., Ltd, Lexicon Pharmaceuticals, Merz Pharmaceuticals GmbH and others.
- Key Diabetic Neuropathy Pipeline Therapies: Suzetrigine, NRD.E1, TRV045, WST-057, ATX01, Cebranopadol, AJH-2947, LX9211, NT 201, and others.
Dive deep into rich insights for new diabetic neuropathy treatments, visit @ Diabetic Neuropathy Drugs
Table of Contents
| 1. | Diabetic Neuropathy Pipeline Report Introduction |
| 2. | Diabetic Neuropathy Pipeline Report Executive Summary |
| 3. | Diabetic Neuropathy Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Diabetic Neuropathy Clinical Trial Therapeutics |
| 6. | Diabetic Neuropathy Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Diabetic Neuropathy Pipeline: Late-Stage Products (Phase III) |
| 8. | Diabetic Neuropathy Pipeline: Mid-Stage Products (Phase II) |
| 9. | Diabetic Neuropathy Pipeline: Early-Stage Products (Phase I) |
| 10. | Diabetic Neuropathy Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Diabetic Neuropathy Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Diabetic Neuropathy Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the diabetic neuropathy cure research, reach out @ Medication for Diabetic Neuropathy Treatment
Related Reports
Diabetic Neuropathy Epidemiology Forecast
Diabetic Neuropathy Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted diabetic neuropathy epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Diabetic Neuropathy Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key diabetic neuropathy companies, including Novo Nordisk, Ionis Pharmaceuticals, Pfizer, Regeneron, NeuroMetrix, among others.
Diabetic Peripheral Neuropathy Market
Diabetic Peripheral Neuropathy Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key diabetic peripheral neuropathy Market companies, including Helixmith, Lexicon Pharmaceuticals, Glenmark Pharmaceuticals, Regenacy Pharmaceuticals, Pure Green, Vertex Pharmaceuticals Incorporated, Eli Lilly and Company, CSPC ZhongQi Pharmaceutical Technology Co., Ltd., Aptinyx, Lexicon Pharmaceuticals, Lateral Pharma Pty Ltd., SIMR (Australia) Biotech Pty Ltd., WinSanTor, Inc., Daiichi Sankyo, Inc., Avazzia, Inc., among others.
Chronic Pain Associated with Painful Diabetic Neuropathy Market
Chronic Pain Associated with Painful Diabetic Neuropathy Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key chronic pain associated with painful diabetic neuropathy Market companies, including Vertex Pharmaceuticals, Tris Pharma, Apurano Pharmaceuticals, Collegium Pharmaceutical, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn
CONTACT: Contact Us Shruti Thakur [email protected] +14699457679 www.delveinsight.com
 Disclaimer: The above press release comes to you under an arrangement with GlobeNewswire. DailyIndiaNews.com takes no editorial responsibility for the same.
Disclaimer: The above press release comes to you under an arrangement with GlobeNewswire. DailyIndiaNews.com takes no editorial responsibility for the same.