Small Molecule API Market Size to Reach USD 328.03 Billion by 2033, Driven by Rising Demand for Cost-Effective Therapeutics – SNS Insider
Strong R&D investment, advanced manufacturing technologies, and supportive government policies accelerate global API production growth.
Austin, Texas, Jan. 15, 2026 (GLOBE NEWSWIRE) — Small Molecule API Market Size & Growth Analysis:
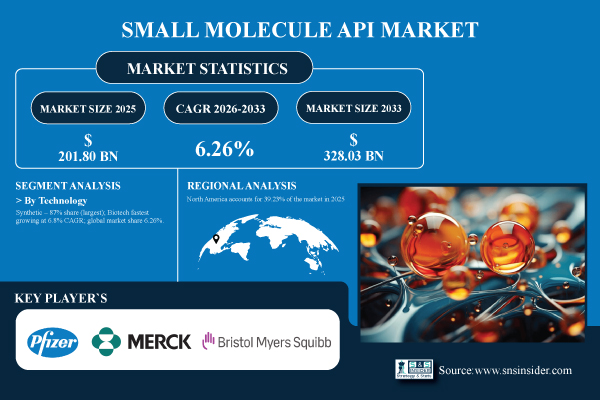
According to SNS Insider, The Small Molecule API Market is estimated at USD 201.80 billion in 2025 and is projected to reach USD 328.03 billion by 2033, expanding at a CAGR of 6.26% during 2026–2033. Global small molecule API market growth is driven by the essential role of small molecule APIs in therapeutics, rising demand for affordable medicines, increased pharma R&D spending, and advances in continuous manufacturing, process optimization, and high-potency API technologies.
The U.S. Small Molecule API Market reached USD 57.79 billion in 2025 and is expected to grow at a CAGR of 6.42% through 2033. Federal incentives, grants, and tax benefits are boosting domestic API production, particularly in oncology and cardiovascular segments. Investments in advanced manufacturing, synthetic APIs, and quality systems are supporting market expansion.
Get a Sample Report of Small Molecule API Market: https://www.snsinsider.com/sample-request/8627
Government Incentives and Policy Support Drive Market Growth:
Governments are encouraging local API manufacturing to strengthen supply chain security. India’s PLI scheme and the Bulk Drug Parks program (INR 69.4 billion / ~USD 800 million) are promoting high-value API production, infrastructure development, and R&D. These initiatives support capacity expansion, technology adoption, and innovation in complex and high-potency APIs.
High Capital and Operational Costs May Hamper Market Growth:
API manufacturing requires high capital investment, GMP compliance, and significant operating costs. Expenses related to raw materials, energy, skilled labor, and regulatory adherence remain key challenges. Compliance with global standards and reliance on imported inputs in Asia, along with high labor and energy costs in North America and Europe, may slow expansion, especially for small and mid-sized players.
Major Players Analysis Listed in the Small Molecule API Market Report are
- Pfizer Inc.
- Merck & Co., Inc.
- AbbVie Inc.
- Bristol-Myers Squibb Company
- Albemarle Corporation
- Boehringer Ingelheim International GmbH
- Cipla Inc.
- Teva Pharmaceutical Industries Ltd.
- Viatris Inc.
- Dr. Reddy’s Laboratories Ltd.
- Aurobindo Pharma,
- Sun Pharmaceutical Industries Ltd.
- Lonza Group AG
- Cambrex Corporation
- GSK plc.
- Novartis AG
- Gilead Sciences Inc.
- Hoffmann-La Roche Ltd.
- Sanofi,
- Novo Nordisk
Segmentation Analysis:
By Technology
Synthetic Small Molecule APIs dominate the market with an 87% share in 2025, attributed to their well-established manufacturing processes, cost efficiency, and widespread use in therapeutic applications across oncology, cardiovascular, and CNS treatments. Biotech-derived small molecules are the fastest-growing segment, with a CAGR of 6.8%, driven by increasing demand for high-potency, targeted therapies and biologically-inspired compounds.
By Manufacturing Type
In-house manufacturing holds the largest share at 60% in 2025, reflecting pharmaceutical companies’ preference for maintaining strict quality control and regulatory compliance. Outsourced manufacturing is the fastest-growing segment, growing at a 7.2% CAGR, fueled by contract development and manufacturing organizations (CDMOs) offering cost-effective production, flexibility, and access to advanced technologies.
By Application
Oncology accounts for the largest share at 30% in 2025, driven by growing cancer prevalence, innovation in targeted therapies, and high demand for chemotherapeutic APIs. Cardiovascular diseases are the fastest-growing segment, with a CAGR of 8.5%, fueled by increasing prevalence of heart disorders, adoption of novel small-molecule drugs, and government initiatives to improve cardiovascular health.
By End-User
Pharmaceutical companies dominate with a 70% share in 2025, leveraging in-house R&D, formulation, and large-scale production capabilities. Biotechnology companies are the fastest-growing end-user segment, expanding at 9.0% CAGR, driven by rising biotech drug pipelines, innovative small-molecule therapeutics, and strategic investments in drug development.
Need Any Customization Research on Small Molecule API Market, Enquire Now: https://www.snsinsider.com/enquiry/8627
Regional Insights:
North America accounts for 39.23% of the market in 2025, with the U.S. leading regional growth. Strong regulatory frameworks, robust pharmaceutical infrastructure, and high R&D investment drive the market.
Asia Pacific holds 24.32% of the global market in 2025 and is the fastest-growing region due to rapid industrialization, rising healthcare demand, and government initiatives promoting domestic API production.
Recent Developments:
- In March 2025, Pfizer announced the expansion of its biologics and small molecule API manufacturing facility in Kalamazoo, Michigan, investing USD 250 million to increase production capacity for oncology and cardiovascular drugs.
- In June 2025, Novartis inaugurated a state-of-the-art API R&D and production center in Basel, Switzerland, to enhance high-potency API production for global markets.
Small Molecule API Market Report Scope
| Report Attributes | Details |
| Market Size in 2025E | USD 201.80 Billion |
| Market Size by 2033 | USD 328.03 Billion |
| CAGR | CAGR of6.26% from 2026 to 2033 |
| Base Year | 2025E |
| Forecast Period | 2026-2033 |
| Historical Data | 2022-2024 |
| Key Segments | • By Type (Synthetic, Biotech) • By Manufacturing Type (In-house, Outsourced) • By Application (Oncology, Cardiovascular Diseases, Diabetes, Central Nervous System (CNS), Others – Anti-inflammatory, Respiratory, Infectious Diseases, Rare Diseases) • By End-User (Pharmaceutical Companies, Biotechnology Companies, CDMOs, Others – Research Institutions, Government Agencies, Academic Labs) |
| Regional Analysis/Coverage | North America (US, Canada), Europe (Germany, UK, France, Italy, Spain, Russia, Poland, Rest of Europe), Asia Pacific (China, India, Japan, South Korea, Australia, ASEAN Countries, Rest of Asia Pacific), Middle East & Africa (UAE, Saudi Arabia, Qatar, South Africa, Rest of Middle East & Africa), Latin America (Brazil, Argentina, Mexico, Colombia, Rest of Latin America). |
Purchase Single User PDF of Small Molecule API Market Report (20% Discount): https://www.snsinsider.com/checkout/8627
Exclusive Sections of the Report (The USPs):
- GLOBAL REGULATORY FRAMEWORK MAPPING – helps you understand how major regulatory authorities such as FDA, EMA, CDSCO, PMDA, and NMPA govern API manufacturing, approvals, and cross-border trade requirements.
- REGIONAL REGULATORY PATHWAY COMPARISON – helps you identify differences in approval processes, documentation requirements, and compliance timelines across regions, supporting informed market entry and expansion strategies.
- API MANUFACTURING COMPLIANCE BENCHMARKS – helps you assess regulatory expectations related to GMP standards, inspections, and quality audits impacting small molecule API production facilities.
- EXPORT–IMPORT REGULATORY RISK ASSESSMENT – helps you evaluate risks associated with changing trade regulations, import/export controls, and regulatory scrutiny affecting global API supply chains.
- THERAPEUTIC SEGMENT–WISE REGULATORY COMPLEXITY INDEX – helps you understand how compliance requirements vary by therapeutic area, enabling prioritization of APIs with lower regulatory barriers or faster approval potential.
- REGULATORY READINESS & MARKET ACCESS SCORE – helps you benchmark API manufacturers based on regulatory preparedness, geographic compliance coverage, and ability to navigate evolving global regulatory environments.
Access Complete Report Details of Small Molecule API Market Analysis & Outlook: https://www.snsinsider.com/reports/small-molecule-api-market-8627
About Us:
SNS Insider is one of the leading market research and consulting agencies that dominates the market research industry globally. Our company’s aim is to give clients the knowledge they require in order to function in changing circumstances. In order to give you current, accurate market data, consumer insights, and opinions so that you can make decisions with confidence, we employ a variety of techniques, including surveys, video talks, and focus groups around the world.
CONTACT: Contact Us: Rohan Jadhav - Principal Consultant Phone: +1-315 636 4242 (US) | +44- 20 3290 5010 (UK) Email: [email protected]
 Disclaimer: The above press release comes to you under an arrangement with GlobeNewswire. DailyIndiaNews.com takes no editorial responsibility for the same.
Disclaimer: The above press release comes to you under an arrangement with GlobeNewswire. DailyIndiaNews.com takes no editorial responsibility for the same.